SALMONELLA SPP. DETECTION - STATUS QUO
The microbial landscape of food production is vast and complex, with certain bacterial groups, such as Enterobacteriaceae and Salmonella spp, often at the forefront of food safety discussions. While Salmonella spp. exhibits an alarmingly low prevalence in factory settings, showing up in a mere 2 to 8 of every 1000 routinely tested samples, total Enterobacteriaceae (TEB) counts can be significantly higher.
Unfortunately, the absence of clear data linking TEB counts to Salmonella in environmental samples has been a sticking point for researchers and industry experts. This ambiguity was further emphasized by the Scientific Panel of EU, which unequivocally concluded that establishing a direct correlation between Enterobacteriaceae and Salmonella spp. is not currently possible.
Needle in a haystack: only 1-4 out of 500 EM samples are positive for Salmonella spp

This low number may create a false sense of safety. Salmonella disease outbreaks are among the most common pathogens, partly due to the lack of quick, reliable prevention and rapid corrective action screening methods.
N-LIGHTTM SALMONELLA TEST KIT - THE FIRST INDICATOR FOR SALMONELLA SPP.
N-Light™ Salmonella Risk is a test kit designed not only for detecting Salmonella spp. Instead of detecting Salmonella spp., it also targets a small selection of closely related bacteria (such as Citrobacter, Klebsiella and Enterobacter) that share specific metabolic and genetic markers.
SALMONELLA RISK – INDICATOR TEST
Positive Salmonella Risk does not mean finding exclusively Salmonella spp. Instead, it is an indicator – a powerful tool revealing increased understanding that a positive result from Salmonella Risk doesn’t necessarily confirm the presence of Salmonella spp, which is crucial.
Salmonella comes from contaminated food and water. This tool identifies an increased likelihood of contamination, allowing for timely interventions and preventive actions to ensure food production safety.
WE TRANSFORM HOW PATHOGEN TESTING AND SALMONELLA DETECTION IS DONE
Environmental monitoring around food production is crucial to avoid pathogen contamination. NEMIS novel N-Light™ Salmonella Risk test kit is the first reliable method that enables lab-free testing and puts you as a food producer in control of your quality management process.
On-site
lab-free testing
safe
closed system from sampling to detection
rapid
take action after only 24 hours
convenient
digital results in 5 simple steps
easy-to-use
anyone can do it
cost-effective
test more for the same budget

WITH NEMIS, YOU ARE IN CONTROL
PIONEERING PHAGE TECHNOLOGY
The patented AquaSpark™ technology powers the N-Light™ tests. The light reaction emitted from highly sensitive chemiluminescent An enzyme specifically triggers molecules. All Salmonella serovars and closely related bacteria occupy the same environmental niche. The robustness of the test is further enhanced by NEMIS proprietary enrichment broth containing phages. Phages oare viruses which Kill competing microflora but are harmless or even beneficial to humans. Test results above the detection threshold indicate an increased risk for the presence of Salmonella pathogen.

Phages kill present microflora but not Salmonella or bacteria occupying the same niche
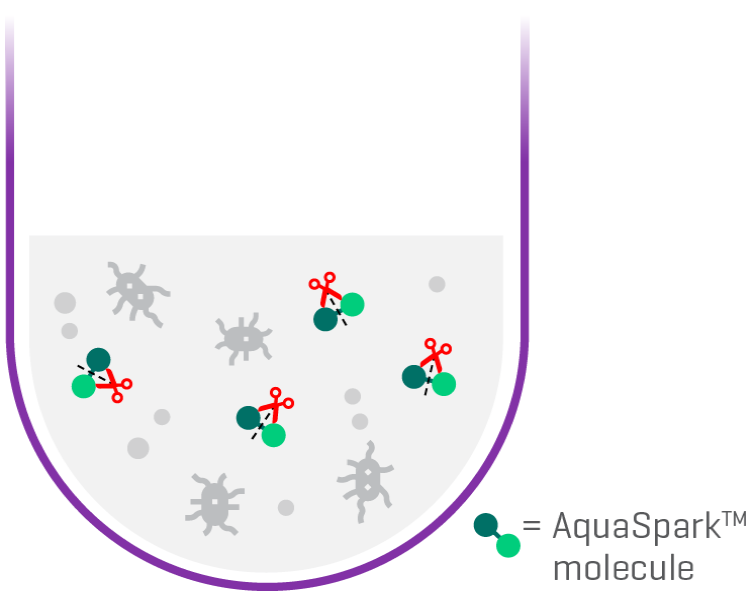
The targeted enzyme breaks the AquaSparkTM molecule
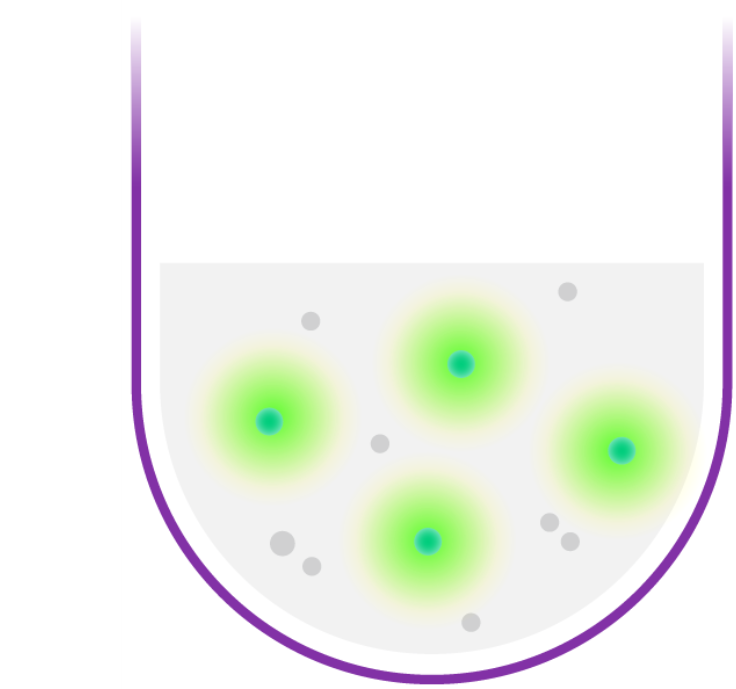
This results in the emission of light, which is then measured
THE BIOSAFETY CAP FOR SAFE ON-SITE ENRICHMENT VALIDATED AGAINST ISO 6579-1:2017

- No leakage at 95 kPa
- Drop Test 1.2 m
N-Light™ Salmonella Risk Holds an AOAC® PTM℠ Certification

THE SALMONELLA RAPID TEST THAT LETS YOU SLEEP AT NIGHT

Bench Top Luminometer (BTL1)
Benchtop luminometer unit for N-Light™ test tubes incl. accessories (touch pen, AC/DC USB power adaptor, USB power cable, NEMIS data app flash drive, instructions for use)
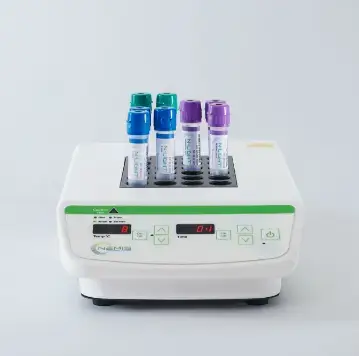
Dry Block Heater 24 (DBH1-24-230V)
Digital dry block heater for 24 N-Light™ test tubes, incl. dry block and power cords (plug types E/F, G and J).

N-Light™ Salmonella Risk: 50 tests + 50 swabs
50 qualitative tests for rapid evaluation of the contamination risk for the foodborne bacterial pathogen Salmonella.
 Switzerland
Switzerland  Italy
Italy  France
France  Spain
Spain  Austria
Austria  Poland
Poland  Belgium
Belgium  Netherlands
Netherlands 