FAQ
This section contains the most frequently requested information about the N-LightTM L. monocytogenes test. Should you have a question that is not addressed here, please contact us at techsupport@nemistech.com.
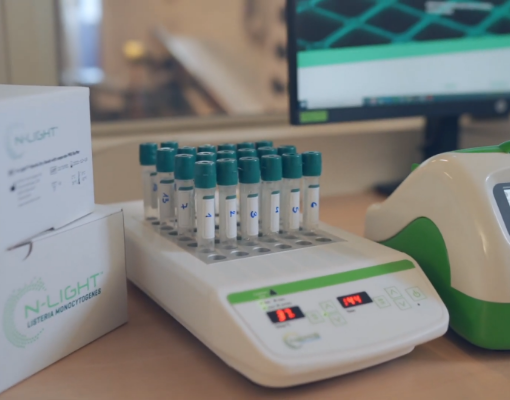
N-LightTM L. monocytogenes is a qualitative test for rapid detection of the foodborne bacterial pathogen monocytogenes. It is intended for use in food processing areas and equipment as part of an environmental monitoring program. Being intuitive, rapid, cost-effective, and lab-free, it offers an affordable solution to scale testing and empower your quality management process.
The test combines benefits from NEMIS bacteriophages and AquaSpark®*, an ultrasensitive chemiluminescent molecule that reacts with an enzyme (PI-PLC) produced specifically by Listeria monocytogenes. In practice, the user follows a simple protocol: collect the sample using a swab, transfer it to the enrichment tube, incubate for 24 hours, and release the AquaSparkTM tablet into the tube. If Listeria monocytogenes are present in your sample, they will initiate a reaction that generates light which is measured by the luminometer, thus yielding a presumptive positive result.
(*) AquaSpark® is a trademark of Ramot at Tel Aviv University
Results can be obtained as early as 24 hours after sampling.
The test is specific for all Listeria monocytogenes, which is the only pathogenic strain for humans. Negative results will be obtained for other Listeria species should they be present in the sample.
The test is sensitive enough to detect L. monocytogenes starting from one single bacterium present in the sample. Good swabbing practice and accurate compliance with the test instructions are required.
No. The detection mechanism relies on the hydrolysis of AquaSparkTM by an enzyme (PI-PLC) that is only expressed by viable Listeria monocytogenes. Therefore, negative results will be obtained for dead bacteria.
YES. Unlike conventional plating methods, the test detects Listeria monocytogenes in their potentially virulent state VBNC. Preliminary research has demonstrated that VBNC cells cleave the AquaSparkTM and can be distinguished from dead cells in an assay format in the laboratory. Further research is ongoing.
The test is designed to be performed on-site without any laboratory infrastructure.
YES, N-LightTM L. monocytogenes has been certified in the AOAC® Performance Tested MethodsSM Program and validated against the reference method ISO 11290-1:2017. AOAC certificate can be downloaded here.
Hot spots that accurately indicate potential product contamination should be preferentially sampled. Please refer to your Hazard Analysis and Critical Control Points (HACCP) plan.
Visual SOP on how to efficiently sample can be found here. Briefly, moisten a swab with specific sterile salt buffer (provided with swabs) and rub it over a 10 x 10 cm area while rotating the swab to collect the maximum amount of sample. Apply a gentle pressure to penetrate biofilms if present. Ensure the swab does not come into contact with anything other than the area of interest to avoid cross-contamination, e.g., from your hands.
Quantitative studies have demonstrated increased amounts of samples collected from environmental surfaces if swabs were pre-moistened (PMID: 20392914; e105786). Only pre-moistened swabs were evaluated by AOAC and validated by NEMIS. Wet area sampling with dry swabs should be re-validated at the factory site.
We recommend starting the test immediately after sampling. Otherwise, samples can be stored at 4 °C and tested within 4 hours, guided by the ISO 18593:2018 norm.
In case of a presumptive positive, you can either run a cultural confirmation or send the tube to an external laboratory using a UN3373 sending procedure. In principle, the N-LightTM L. monocytogenes test can also be confirmed via PCR or immunogenic reactions.
Contact us for a list of validated confirmation methods.
For negative control, please follow these steps:
- Activate one N-LightTM monocytogenes test containing only the enrichment broth without sample. Shake until the tablet is dissolved.
- Incubate at 37°C for 10 minutes (NEMIS Incubator).
- Test with the luminometer 10 minutes after activation.
- Results up to 10.000 RLU are acceptable. In case of larger values, contact techsupport@nemistech.com for further guidance
Positive control: Development in progress.
AquaSparkTM are dioxetane derivatives bearing substrates that specifically react with targeted enzymes. Being fully synthetic molecules, the platform offers a wide array of variations that allow rapid detection of enzymatic activities, amongst other applications. Additional information about AquaSparkTM technology can be found here.
Check out our other publications.
To offer an ideal culture medium for Listeria monocytogenes leading to superior sensitivity and specificity of the test, NEMIS proprietary enrichment broth contains selective substances including bacteriophages as well as stimulating nutrients. Its unique formulation inhibits the growth of competing bacteria and makes the test more reliable in challenging environments.
The new neutralizing agent is a simple yet powerful addition to N-Light range of rapid tests. By moistening the swab with the neutralizer prior to sample collection, NEMIS customers can now overcome the potential barriers posed by disinfectants and achieve more accurate and dependable testing outcomes.
Benefits of N-Light Neutralizer:
- Enhanced Bacterial Survival: When using our neutralizing agent, a significantly higher number of bacteria survive in challenging conditions on the swab compared to non-neutralizing solutions. This results in a higher starting inoculum in the testing tube, ensuring more accurate and reliable test results.
- Improved Bacterial Health: Our neutralizing agent keeps the bacteria healthier and in better shape throughout the testing process. Thus, they can begin growing sooner and more vigorously. This leads to a substantial increase in bacterial quantity after 24 hours of incubation.
- Extended Swab Storage: Unlike traditional buffers, our neutralizing agent allows for longer storage of swabs before analysis. Bacteria tend to slowly die in PBS due to the absence of nutrients. In contrast, our neutralizing agent provides the necessary nutrients for bacterial growth, allowing the bacteria to thrive even during storage. This feature enhances convenience and flexibility in testing procedures, without compromising the integrity of results.
| Maximum time of storage of samples at room temperature before incubation | |||
| Sample type | PBS | BPW | N-Light neutralizer |
| swab with bacteria kept in transportation tube | up to 48 h | up to 48 h | up to 72 h |
HOW TO USE THE
N-LightTM Listeria MONOCYTOGENES
 Switzerland
Switzerland  Italy
Italy  France
France  Spain
Spain  Austria
Austria  Poland
Poland  Belgium
Belgium  Netherlands
Netherlands 
