Empowering production to embrace food safety at the line 24/7
Mobility, global trade, and population growth are accelerating the spread of pathogenic microorganisms. Simultaneously, health authorities are steadily tightening regulations and have acquired the technical means to trace sources of food contamination from patients back to producers. While food producers bear a steadily increasing risk due to improved outbreak investigation and consumer demand changes influenced by media coverage, effective tools to combat this risk are lacking. Conventional pathogen tests are not suitable for the food processing industry’s complex, fast-paced environment.
COVID has introduced a paradigm shift towards preventive screening. Many countries started: (1) Testing too late so the infection spread unnoticed. (2) Testing too little so they did not find infection hotspots. (3) And relying on overwhelmed central laboratories. The key learning: early mass-testing using self-tests for home use whenever possible. This is the very solution the food industry is lacking today.
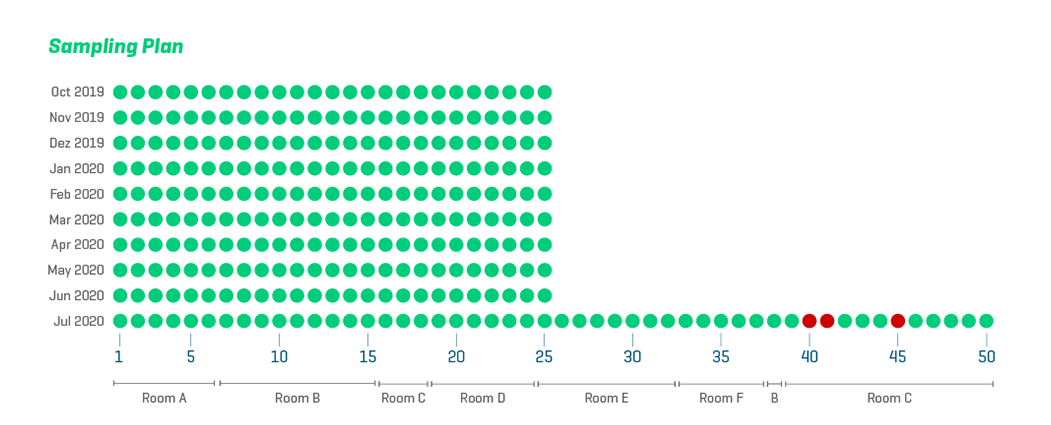
The detection of any pathogen may lead to the destruction of whole batches for consumer safety, resulting in unsustainable food waste. It is common practice to heavily rely on end-product testing, thus testing too late. Additionally, most producers only test single-digit food samples from tons of food which is far from representative. In summary, testing today is done not only too late and in the wrong places but also too little as well. Why does the food industry not perform more controls at potential contamination sources? Because microbiology testing requires full-scale laboratories.
And this is what we at NEMIS are changing!
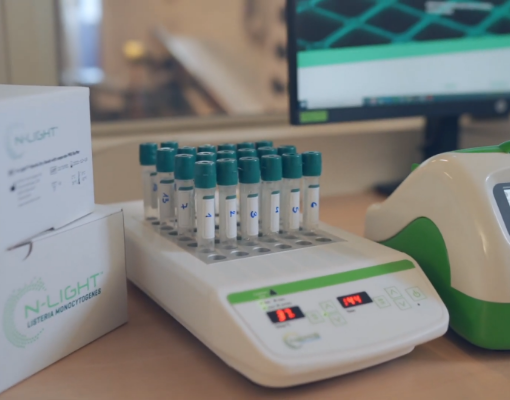
How to use the
N-LightTM listeria
MONOCYTOGENES
Bring your environmental monitoring to the next level
Fast, flexible and mobile for wherever you need it
While laboratory-based genotypic solutions such as PCR may be precise, relying on laboratory services can be costly. Limited budgets impose a great constraint on the volume of samples that can be analyzed, statistically reducing the likelihood of detecting pathogen hotspots. On-site tests enable users to test frequently and extensively as our rapid tests can be performed by anyone anytime. This increases the flexibility regarding risk-based definitions of both sample spots and volume.
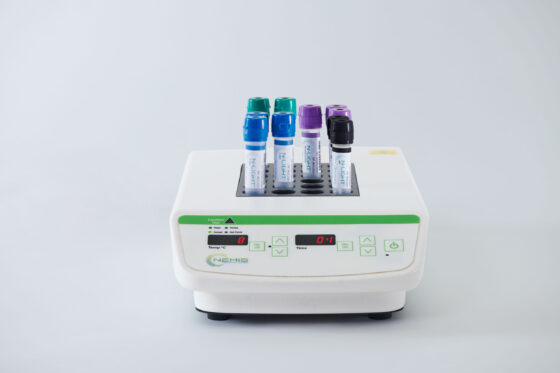
Easy and safe to use, tamper-proof and accessible for everyone
When relying on the services of external laboratories, food producers usually wait multiple days before receiving actionable data. With our on-site rapid tests, the first results are available within 24 hours of sampling, granting producers crucial time to prevent the spread of pathogens throughout the production environment.
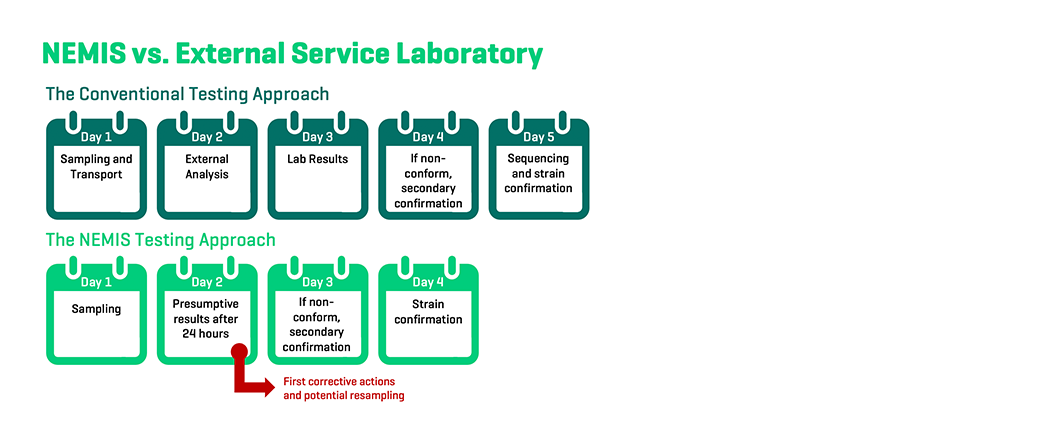
Affordable, accurate and high-frequency testing whenever needed
While the advantages of an effective on-site screening program are evident, most rapid tests fall short in terms of specificity and sensitivity as competing microflora challenges the reliability of these methods. NEMIS leverages pioneering phage technology to selectively enrich and detect only targeted pathogens, consequently significantly outperforming the chromogenic competition in the market. Read more about our phage technology.
Contact us for more information!
On-site
No lab requiredSafe
Closed system from sampling to detectionRapid
Take action after only 24 hoursConvenient
No sample preparation requiredEasy-to-use
No highly qualified staff neededCost-effective
Enables mass testing
With NEMIS, you are in control
Managing food safety risks for L.mono, Salmonella, E.coli, and ATP.
 Switzerland
Switzerland  Italy
Italy  France
France  Spain
Spain  Austria
Austria  Poland
Poland  Belgium
Belgium  Netherlands
Netherlands 